Lauric Acid Dihydroxyethyl Imidazoline traces its roots back to the mid-twentieth century, a period when scientists ramped up efforts to modify natural fatty acids for better surfactant and corrosion inhibition properties. Practically speaking, the marriage of lauric acid—an abundant component in coconut oil—with imidazoline derivatives offered answers to both performance and cost challenges in industrial chemistry. The early adoption by oilfield and cleaning industries showed how necessity and tinkering with molecular structures walked hand in hand. Over decades, improvements in purity and yield reflected pressures from both environmental regulations and production demand. I remember reading archived journals that documented the transition from basic lab syntheses to scalable processes as markets pushed for better product reliability. Back then, the transformation felt much more like art than routine batch chemistry.
Diving into what makes Lauric Acid Dihydroxyethyl Imidazoline tick, its profile reveals a compound capable of handling both hydrophilic and hydrophobic challenges. The molecule brings together the 12-carbon fatty acid tail from lauric acid and the functional heads of imidazoline and hydroxyethyl groups. These features help it act as a bridge between oil and water, which explains its popularity in emulsification, antistatic, and anti-corrosion applications. Industry users look for materials that don't just perform one task but handle several demands in one sweep. This compound proved itself over time, standing out for its cost-effectiveness and versatility, as much as its ability to meet specific environmental or technical hurdles that alternatives couldn't address.
The compound typically appears as a yellowish, viscous liquid at room temperature, bringing a saponifiable base combined with a stable heterocycle. The imidazoline ring gives chemical robustness, standing up to moderate acid and base exposure during typical process cycles. With a melting point usually below 30°C and ready solubility in alcohols and glycols, it accommodates various processing requirements. Surface activity stays high even in hard water, resisting precipitation or loss of function, which often annoys operators using other amines. I once handled material analysis for a customer; even slight changes in pH or ionic strength hardly dented the performance, a rare property for surfactants derived from natural fats. Boiling point stretches past 200°C, though most real-world use cases don't push it that high. This thermal stability lets the compound hang in there during hot-cycle cleaning operations, something users in the field appreciate every day.
Commercially available Lauric Acid Dihydroxyethyl Imidazoline usually comes specified by active content percentage, color, and the level of unsaponifiable matter. Companies keep an eye on impurities like free amine or unreacted lauric acid, as both can trigger undesirable foaming or stability headaches. Product labeling typically carries clear CAS numbers, batch codes, purity levels, and recommended shelf life, but also hazard and precautionary statements required by updated GHS labeling standards. I recall confusion in a warehouse once due to indistinct lot records; concrete, regulatory-compliant labeling helped clear up cross-contamination doubts. In the age of digital traceability, simple QR codes on these bulk drums save buyers a lot of fuss tracking transport history and analysis results.
Manufacturers rely on a straightforward yet sensitive process—condensing lauric acid or its methyl ester with diethanolamine under controlled conditions. Initial heating in an inert atmosphere drives off water, so the imidazoline forms cleanly without too much polymerization. Catalysts help tune the reaction, as batch temperature jumps quickly past safe limits without watchful management. I've spent enough late nights in pilot plants to know that a missed temperature setpoint can yield a batch of gelled disappointment instead of usable product. Care in handling raw materials, controlling water content, and quenching the reaction at the right moment determines the quality, purity, and ease of downstream blending. Process engineers and chemists need a tight grip on times, temperatures, and reactant ratios to keep production within spec.
Lauric Acid Dihydroxyethyl Imidazoline offers a reactive imidazoline ring, which opens doors for further modification. Chemists attach various functional groups for compatibility tweaks, enhanced wetting, or tailored corrosion resistance. In the lab, this derivative often gets sulfonated or quaternized, which flips its charge and boosts solubility in problematic brines or high-chloride environments. Real-world modifications help tailor the product for textile softening, antistatic coatings, or even specialty biocidal applications. I've seen teams work on grafting ethoxylates onto this backbone to finesse foam profiles or dispersibility in different finished products. Flexibility here makes the compound a workhorse rather than a one-trick performer.
Industry catalogs and safety data sheets throw around a handful of names for Lauric Acid Dihydroxyethyl Imidazoline: typically, "Cocoyl Dihydroxyethyl Imidazoline", "2-(2-Hydroxyethyl)imidazoline", and "C12 Dihydroxyethyl Imidazoline". You might catch it under tradenames that signal brand loyalty in the oilfield or cleaning sector, but the core chemical identity stays the same. I’ve witnessed confusion among buyers who received what they thought were different products from two suppliers—turns out, both carried the same structure, only packaged differently, which reinforces the value of cross-referencing by CAS number rather than marketing claims.
Handling Lauric Acid Dihydroxyethyl Imidazoline calls for a practical safety mindset. The compound can irritate skin and eyes, especially at high concentrations or during handling of raw intermediates. Companies with boots-on-the-ground experience favor closed transfer systems and personal protective gear—nitrile gloves, goggles—and careful ventilation in blending rooms. Spills gel into slick puddles, so clean-up protocols focus on containment, dilution, and neutralization where needed. Wastewater pre-treatment topples discharge risks, making environmental audits less stressful. Strict adherence to REACH and GHS keeps plants running without compliance headaches. Training matters—I've seen near-misses when contractors missed chemical PPE briefings, reinforcing the point that safety always dances with repetition and vigilance, not just good intention.
Lauric Acid Dihydroxyethyl Imidazoline thrives across industries. Oilfield teams pump it for corrosion inhibition, where tough environments hammer steel surfaces. In textile softeners, it brings that sought-after hand feel, while coatings and cleaning products appreciate its foaming and emulsifying strength. It slips into antistatic applications, helping electronics and plastics keep dust and charge down. In each field, reliability and synergy with other ingredients matter more than theoretical performance. A customer once told me their shift switched to this imidazoline blend because it solved not just technical snags but also delayed downtime and simplified clean-in-place steps—real benefits, not just lab statistics.
Research into this imidazoline derivative doesn’t slow down. Universities and formulation labs keep spinning up projects around sustainable sourcing—how to pull lauric acid from upcycled or traceable sources, avoiding deforestation and supply disruptions common with coconut and palm. Alternative synthesis tries out lower temperatures or green solvents to cut batch cycle times and reduce plant emissions. Advanced analytical profiles help track trace impurity effects, especially for products touching sensitive consumer markets. Collaborations between producers and downstream users encourage practical bench-to-plant feedback. I've worked with teams who run quick-and-dirty emulsion trials right after a batch tweaks, which cuts down cycles of missed expectations. A focus on regulatory trends means R&D pushes for biobased status, skin compatibility, and safer blends, to greenlight adoption in tomorrow’s applications.
Toxicological studies on Lauric Acid Dihydroxyethyl Imidazoline flag moderate irritation at high concentration, with no clear evidence, to date, of chronic toxicity in standard workplace exposure. In aquatic toxicity assays, breakdown products draw more attention than the parent molecule, so effluent management remains a regular talking point for plant managers. Reviewing reports and workplace logs, allergic reactions turn up rarely, mostly tied to repeated, careless contact rather than routine, controlled exposure. Still, comprehensive toxicological panels take time and transparency—no shortcut matches real data on humans and ecosystems. Constant literature review and voluntary testing close information gaps, supporting safer industrial and consumer product claims.
Lauric Acid Dihydroxyethyl Imidazoline faces the same pressures as many specialty chemicals—better sustainability, regulatory hurdles, and customer expectations for reduced irritation and environmental footprint. Shifts toward biobased sourcing and smarter chemical recycling promise lower long-term costs and easier compliance with coming green regulations. As digital tracking tightens, blockchain and smart labels will make provenance and ingredient safety easier to prove. Applied research will likely strengthen protein compatibility, broaden antimicrobial claims, and slice carbon intensity from manufacturing. If anything, customer and regulatory scrutiny will help keep product development honest and focused, steering this class of surfactant toward safer, more circular, and more socially acceptable futures.
Lauric Acid Dihydroxyethyl Imidazoline isn’t one of those chemicals people recognize from the label on a shampoo bottle or a laundry soap commercial. Chemists know it for an entirely different reason. It helps create products with staying power and reliable performance. I stumbled into it years ago, working my first job mixing cleaners for an industrial supplier. We rarely talked about ingredients with names this long, but the lab folks would always point out that certain additives did the heavy lifting.
Walk through any maintenance supply closet in a factory or a hospital. The bottles of degreasers, antistatic sprays, and rust preventatives have something in common—they need surfactants to do their job. Lauric Acid Dihydroxyethyl Imidazoline sits in the category known as imidazoline surfactants. This type works as an emulsifier, which means it helps oil and water blend. The result? Oil-based grime comes off with water-based cleaners. That’s not minor. Anyone who’s tried to wash greasy hands in cold water without soap knows what I’m talking about.
People in textiles use it for softening fabrics. Paper mills put it in their water treatment process. Lubricant makers rely on it to keep machinery protected under strain. There’s a reason for the cross-industry popularity. Based on field experience, these surfactants plug holes in product performance. They cover gaps between water-loving and oil-loving ingredients, delivering a solution that genuinely works.
Chemicals like this don’t travel alone; they come with questions on human and environmental impact. Overuse or mishandling of industrial surfactants can leave lasting marks on waterways, wildlife, and wastewater systems. Skin irritation sometimes happens if workers skip gloves. One thing I learned from safety training: respect goes a long way in a chemical storeroom. Knowledge and routine risk checks keep everyone out of trouble.
The European Chemicals Agency lists detailed safety profiles and has regulations about how much can be added and how waste gets treated. Plants with good environmental stewardship record every gram used and send wastewater to proper treatment. Individuals at home won’t find this compound in off-the-shelf products in the same way, which helps reduce accidental overexposure.
The talking point that sticks with me: better alternatives exist, but few have the same blend of performance and cost. Companies sometimes switch to greener options, but these rarely match the same results for all applications. Researchers search for plant-based alternatives, focusing on biodegradable surfactants. Some startups experiment with coconut-derived imidazolines to improve biodegradability.
Industry can work toward safer processes by choosing formulas with reduced toxicity and boosting employee education. Regulatory pressure and consumer advocacy also drive change, but at the shop floor, it still comes down to following best practices and reviewing ingredient lists. Staying informed helps both workers and companies make smarter, safer choices.
Most people don’t think about what keeps machines running smoothly, keeps floors spotless, or makes shirts less scratchy. Yet, behind all those day-to-day comforts stand specialty chemicals like Lauric Acid Dihydroxyethyl Imidazoline. With a better understanding, people can weigh the trade-offs between effective performance and responsible use—right in the spots where it matters the most.
Lauric acid dihydroxyethyl imidazoline falls into a chemical group that often shows up in detergents and some industrial cleaners. On the label, it may look like a tongue twister, but companies pick this compound for a reason—it helps with foaming, cleaning, and can even act as an anti-static agent. Its chemistry gives it a greasy, “slippery” characteristic, and it usually gets used in stuff that needs a little help breaking down oils and dirt.
People want reassurance about what touches their skin, especially as allergic reactions and irritations seem more common than ever. The science behind lauric acid dihydroxyethyl imidazoline shows that direct, prolonged contact can irritate the skin. Industry watchdogs and regulatory groups, like the European Chemicals Agency (ECHA) and the U.S. Environmental Protection Agency (EPA), list it as a potential irritant. Formulators rarely use it in high concentrations for products designed to stay on your skin, such as lotions or creams.
Accidentally getting it on your hands from diluted soap in a public restroom or from handling industrial cleaning agents probably won’t cause an instant hazard. Frequent, repeated exposure tells a different story, though. Dermatitis—red, itchy skin—has been reported in workers dealing with it in manufacturing jobs. This matches what’s known from similar imidazoline-based substances. Sensitization sometimes follows repeated exposures, leaving a person with an allergic response that only worsens with more contact.
Dermatology journals and chemical safety data sheets highlight the risk in certain professional settings, including janitors, food processing workers, and lab technicians. In these cases, even gloves sometimes lose the war against skin irritation if little spills happen day in and day out. Anecdotally, folks in those jobs sometimes mention dry, cracked fingers and hands—symptoms that clear up when contact stops.
The Cosmetic Ingredient Review panel, which weighs in on these matters, puts up safety guidelines: rinse-off products can use it up to certain concentrations, but anything meant to sit on the skin stays well below those levels or skips it altogether. Household products with this ingredient tend to go through a rinse cycle, washing most of it away with suds and water. That practice lines up with what dermatologists recommend for any known irritant.
From the experience of handling chemicals as a science student and hobby tinkerer, gloves and a sink stay close by. Accidents happen fast; a splash or a foam bubble pops against the arm. Rinsing right away usually prevents problems. Over the years, seeing coworkers skip gloves or ignore a label led to plenty of unnecessary rashes. Reading the fine print helps, but most people just want simple advice: if you spot lauric acid dihydroxyethyl imidazoline in the ingredient list, stay cautious, especially if you already have sensitive skin.
Regulation and transparency play a big role in keeping exposure in check. Companies following European rules filter out much of the risk, keeping concentrations low and safety data accessible. For anyone at home, reading labels and choosing products meant for skin contact from reputable sources reduces the odds of trouble. In the workplace, safety managers should keep up with Material Safety Data Sheets and provide practical alternatives or proper gear.
Switching to gentler, plant-based ingredients or taking a look at old-school, simple soaps makes sense if skin flare-ups have become routine in your household. The big takeaway: lauric acid dihydroxyethyl imidazoline works hard in the right setting, but skin deserves a thoughtful approach—especially if that ingredient lands in your daily routine.
Few chemicals stand up to soap scum, oil, or grease quite like Lauric Acid Dihydroxyethyl Imidazoline. This molecule acts as a workhorse in things like dish soap, hand cleansers, and industrial degreasers. It pulls oil and dirt off surfaces, lathers up smoothly even in hard water, and leaves no weird residue behind. My years working in facility management taught me to appreciate substances that actually deliver—not just on promises, but on hard surfaces caked with grime. Watching a crew knock down engine oil in a machine shop thanks to a cleaning agent loaded with this ingredient, you see why factories stick with it.
Not all surfactants play nice with skin, but products containing Lauric Acid Dihydroxyethyl Imidazoline regularly show up in hand soaps for nurses or line workers. There's a reason for this. Compared to harsher detergents, this compound feels mild: it doesn’t strip away natural oils, so hands stay less irritated after dozens of washes. Various toxicology panels and years in healthcare confirm its safety for repeated exposure. Choose the right base, and the scent and feel can be surprisingly pleasant. That’s why you’ll see it in “gentle” soaps and even baby shampoos.
People worry—for good reason—about cleaning chemicals going down the drain. Lauric Acid Dihydroxyethyl Imidazoline breaks down over time in water, helping to shrink its environmental footprint. According to multiple environmental assessments, this substance degrades more easily than a lot of older formulas. Working in municipal waste and water systems, I came to trust cleaners with these sorts of ingredients: less anxiety about bioaccumulation or nasty persistence in the food chain. Still, responsible use and proper wastewater management make a big difference—no single ingredient wipes out all risks.
Besides cutting grease, this chemical also helps prevent rust and corrosion. In machinery workshops or food processing plants, a product that both cleans and protects saves real money. The imidazoline ring structure forms a thin layer that clings to metal, fending off moisture and salt spray. I’ve seen equipment last longer and run more reliably when the cleaning solutions include these active agents. It’s not magic—just chemistry doing its job day after day.
Products based on Lauric Acid Dihydroxyethyl Imidazoline don’t usually give off strong fumes or trigger nasty reactions in workers. Compared to ammonia or bleach solutions, employees complain less about headaches, rashes, or breathing trouble. Cleaner air and healthier hands translate to fewer sick days and better job satisfaction. I’ve witnessed janitorial staff reach for these soaps voluntarily, even if stricter PPE isn’t required. That says a lot about comfort and peace of mind on the job.
Nothing’s perfect. Runoff still needs treatment, and some users may find this compound isn’t “green” enough for their standards. Focusing on low-impact formulas, using the right dose, and investing in wastewater clean-up help the most. Brands that communicate openly about sourcing and safety win more trust. My experience shows companies that push for better supplier transparency and regular in-house testing can spot problems—before they hit the public or the environment.
Cleaning up isn’t glamorous, but it powers industries and keeps spaces safe for everyone. Lauric Acid Dihydroxyethyl Imidazoline brings together cleaning punch, safety, and a bit of environmental sense. Whether it’s a hospital ward, a batch of clean baby bottles, or a garage packed with greasy tools, the right ingredient choices mean healthier workers, longer-lasting equipment, and less impact on the world outside.
Lauric Acid Dihydroxyethyl Imidazoline looks like a tongue-twister straight out of a chemistry textbook. Yet, people around the world rely on it as a surfactant and corrosion inhibitor in many oils and cleaning products. I remember the first time I worked with this substance in an industrial supply room—the pungent smell lingered, and after an accidental spill, the lesson was clear: you can’t shrug off proper storage, even for chemicals that don’t seem volatile at first glance.
This compound holds up best in cool, dry areas. Temperatures between 10°C and 25°C keep it from breaking down or releasing unpleasant odors. Excessive heat speeds up unwanted chemical changes, sometimes forming sticky residues that render it useless for precise blends.
Letting moisture creep into storage spaces causes clumping and strange reactions. During summer months, a little extra care—maybe a dehumidifier or moisture-absorbing packets—goes a long way. One rainy season, I watched entire drums of chemicals spoil thanks to leaky roofs and overlooked container lids. Dryness isn’t just a suggestion, it’s the difference between product and waste.
Storing Lauric Acid Dihydroxyethyl Imidazoline means using tightly sealed, chemical-resistant containers. No one wants to deal with rusty steel or cracked plastic. Polyethylene and high-grade stainless steel stand up to both the substance and the test of time. If you’ve ever broken open a drum to find hardened lumps or rusty streaks, it’s clear—quality containers prevent a world of headaches.
Direct sunlight seems harmless, but it speeds up chemical change. Store containers away from windows. Ultraviolet rays creep in, especially in facilities with skylights or glass panels, breaking down the chemical’s core structure. Shielding catch-all shelves or adding simple covers pays off. I’ve learned to check for unexpected “hot spots” near windows.
Well-ventilated storage rooms stop fumes from accumulating. People who handle this compound all day value fresh air—nobody enjoys headaches or worse. Labeling every container in large, legible print with the substance name, batch date, and hazard warnings helps everyone avoid mistakes. Proper signage should not be a luxury.
Even with strict storage, spills happen. Absorbent pads, gloves, and goggles must always stay within arm’s reach. Once, we seriously underestimated how much mess a single leaky drum could make and spent hours cleaning it up—dust masks and heavy-duty soap saved the day. A bit of planning saves nerves and keeps the workspace safe.
Routine inspections catch problems before they spread. Checking containers for cracks, leaks, or bulges helped us spot early signs of trouble. Safe storage doesn’t end once the chemical lands on the shelf. It’s an ongoing practice that keeps workers healthy, products effective, and the local environment protected.
Storing Lauric Acid Dihydroxyethyl Imidazoline seems simple until trouble pops up. Experienced staff, sturdy containers, climate control, and clear labels keep problems small. Health, safety, and cost management all win when companies pay attention to these practical details. Investing in proper storage isn’t just smart—it’s the responsible thing to do for people and planet alike.
Lauric acid dihydroxyethyl imidazoline pops up in plenty of personal care products, mainly in shampoos, hand soaps, and some industrial cleaners. It belongs to a class of surfactants built for cleaning, foam boosting, and emulsifying. Many people go about their routines without even realizing this compound plays a behind-the-scenes role in daily hygiene.
Putting faith in any ingredient deserves at least a basic check-up for risks. A search through scientific literature shows that lauric acid dihydroxyethyl imidazoline generally holds up as safe in rinse-off products, but there are a few notes worth paying attention to.
People with sensitive skin are more likely to notice effects such as redness, dryness, or itching. Reports in cosmetic toxicology journals point out that, like most surfactants, this compound can strip away a bit of the skin’s natural oils. This sometimes stirs up mild irritation, especially if someone already deals with eczema or has a fragile skin barrier.
Eyes take to this chemical less kindly, which isn’t out of the ordinary for this category. Direct contact with the eyes can result in stinging and discomfort, much like getting shampoo in your eyes. Over the years, working in commercial cleaning, I’ve seen staff react with sore, red hands after using industrial soaps packed with these sorts of surfactants for hours on end. Gloves usually deal with that, but occasional flare-ups still happened, pointing to the need for moderation.
International safety groups, such as the Cosmetic Ingredient Review (CIR) Expert Panel, have looked at this family of compounds. Their findings show low toxicity when used in standard concentrations. In rare cases, patch tests highlight allergic contact dermatitis, but these are not common events. For the average consumer, normal use barely registers on the risk scale.
Companies keep formulas within concentrations that minimize negative effects. Higher concentrations, like those in industrial settings, draw more complaints about skin dryness or cracking. My experience in janitorial supply taught me to pay attention to the dilution instructions. Full-strength is often too harsh without gloves.
True allergies crop up much less often than irritation. The mechanism typically revolves around an overactive immune response to persistent exposure. Think of workers who use strong soaps every day and suddenly develop rashes or bumps on their hands. Regular folks using typical hair or hand soap rarely see reactions unless allergies run in the family or skin is highly reactive already.
Simple steps protect most people from the downsides. Rinsing thoroughly, avoiding eye contact, and using fragrance-free or sensitive-skin formulas go a long way. If irritation does show up, swapping to a milder cleanser often solves the problem. For those with a history of allergic reactions, patch testing new products can catch issues before they start.
Some brands work to tweak their mixes, lowering concentrations and adding moisturizing agents like glycerin or panthenol. This approach keeps the cleaning power intact and gives the skin a better chance at staying comfortable, even with regular use.
The risk tied to lauric acid dihydroxyethyl imidazoline centers on overuse or unsuitable skin types. Choosing gentler options, knowing your own sensitivities, and following proper product use tips the balance toward safety every time.
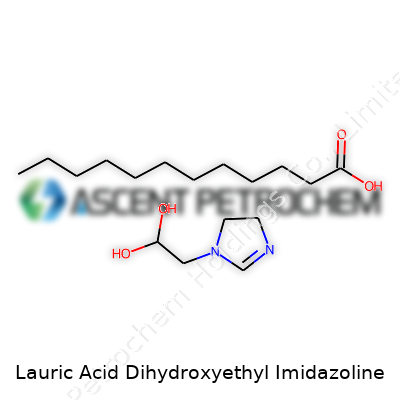
| Names | |
| Preferred IUPAC name | 2-(2-hydroxyethyl)-1-lauryl-4,5-dihydroimidazole |
| Other names |
2-(2-Hydroxyethyl)imidazoline-1-lauric acid Lauric diethanolamide imidazoline Imidazoline laurate |
| Pronunciation | /ˈlɔːrɪk ˈæsɪd daɪˌhaɪdrɒksiˈɛθɪl ɪˌmɪdəˈzoʊliːn/ |
| Identifiers | |
| CAS Number | 26590-05-6 |
| Beilstein Reference | 2884934 |
| ChEBI | CHEBI:134662 |
| ChEMBL | CHEMBL1544851 |
| ChemSpider | 60805 |
| DrugBank | DB11267 |
| ECHA InfoCard | 17a2a0e2-f6e6-4f80-b205-ec6cd3656c25 |
| EC Number | 263-057-9 |
| Gmelin Reference | 135157 |
| KEGG | C19643 |
| MeSH | D02.455.326.271.585.500 |
| PubChem CID | 10461 |
| RTECS number | OJ5775000 |
| UNII | 38D65MG3YN |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID6014332 |
| Properties | |
| Chemical formula | C17H34N2O3 |
| Molar mass | 346.53 g/mol |
| Appearance | White to yellowish solid |
| Odor | Characteristic |
| Density | 0.981 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 3.83 |
| Vapor pressure | Negligible |
| Acidity (pKa) | ~4.8 |
| Basicity (pKb) | 7.8 |
| Magnetic susceptibility (χ) | -62.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4740 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.14 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.4 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | C05AX02 |
| Hazards | |
| Main hazards | Irritating to eyes, skin, and respiratory system |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Avoid breathing dust/fume/gas/mist/vapors/spray. Wash thoroughly after handling. Use only outdoors or in a well-ventilated area. Wear protective gloves/protective clothing/eye protection/face protection. |
| Flash point | 204°C |
| Lethal dose or concentration | LD50 oral rat 3700 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| NIOSH | KN1450000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Lauric acid Imidazoline Fatty acid imidazolines Cocamidopropyl betaine Cocamide DEA |